For a better understanding of how temperature and pressure influence air density, let's focus on a case of dry air. It contains mostly molecules of nitrogen and oxygen that are moving around at incredible speeds. Use our particles velocity calculator to see how fast they can move! For example, the average speed of a nitrogen molecule with a mass of 14 u (u - unified atomic mass unit) at room temperature is about 670 m/s - two times faster than the speed of sound! Moreover, at higher temperatures, gas molecules further accelerate.
As a result, they push harder against their surroundings, expanding the volume of the gas . And the higher the volume with the same amount of particles, the lower the density. Therefore, air's density decreases as the air is heated. Air density equations Air contains a mixture of dry air and water vapor.
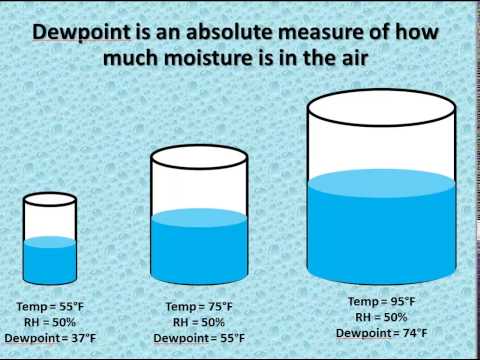
The amount of water vapor is a function of the relative humidity; it is also related to the dew point temperature of the air. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. If you need to calculate the density of dry air, you can apply the ideal gas law. This law expresses density as a function of temperature and pressure.
Like all gas laws, it is an approximation where real gases are concerned but is very good at low pressures and temperatures. Increasing temperature and pressure adds error to the calculation. Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures.
An online air density calculator such as the one by Engineering Toolbox let you calculate theoretical values for air density at given temperatures and pressures. The website also provides an air density table of values at different temperatures and pressures. These graphs show how density and specific weight decrease at higher values of temperature and pressure.
The density of air is usually denoted by the Greek letter ρ, and it measures the mass of air per unit volume (e.g. g / m3). Dry air mostly consists of nitrogen (~78 %) and oxygen (~21 %). The remaining 1 % contains many different gases, among others, argon, carbon dioxide, neon or helium. However, the air will cease to be dry air when water vapor appears.
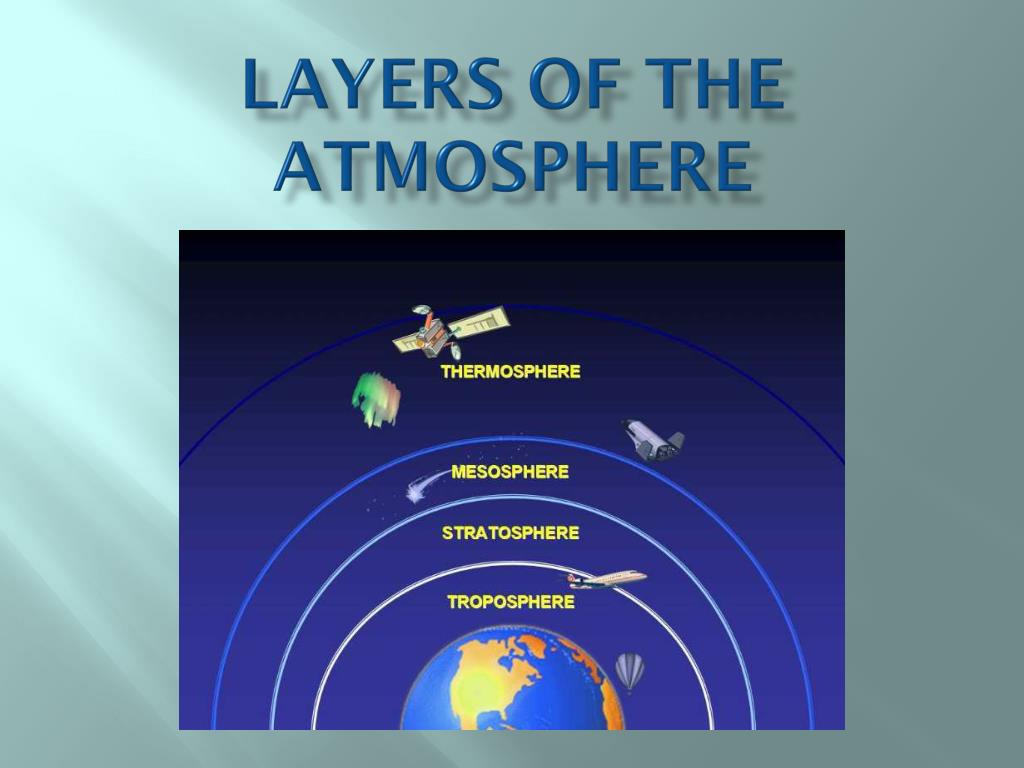
The density of air depends on many factors and can vary in different places. It mainly changes with temperature, relative humidity, pressure and hence with altitude . The air pressure can be related to the weight of the air over a given location. It is easy to imagine that the higher you stand, the less air is above you and the pressure is lower (check out our definition of pressure!). Therefore, air pressure decreases with increasing altitude. In the following text, you will find out what is the air density at sea level and the standard air density.
The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture. In the first calculator, the vapour pressure of water vapour in saturated air at the nominated temperature is calculated and multiplied by the relative humidity to give the actual water vapour pressure. The water vapour pressure is then subtracted from the total pressure to give the pressure of the dry component of the parcel. Densities of the two components are then calculated and summed to give the final answer. We have the initial pressure of the air and then pressure due to the wire column.
Density Of Air At 20C And 1 Atm We need to find the final pressure which is equal to the pressure of the air plus pressure due to the water column. We're just going to convert that to kilograms over meters Q. And then we're going to calculate the pressure due to the water column using the formula density times acceleration due to gravity of tight.
So note the units on the that's the acceleration due to gravity and the height. These will cancel out to give us the units equivalent to the pascal. So you have to make sure that these are the units that you have on new values in order to get Depression and Pascal is then convert that the killer Pascal's by divided by 1000. So add this to P one the initial pressure or the barometric pressure.
Then we're going to just convert the pascal's two atmospheres first because the barometric pressure was one atmosphere. And that way we can add the pressures and get the new pressure. And we're going to use Boyle's law to solve from Youtube. So then we solve for V. Two which gives us the volume of compressed air.
So the mass doesn't change because the air is compressed so we can just take the original mass of one leader and divided by the new volume, Then we can get the new, etc. This phenomenon is wonderful for those who enjoy helium-filled balloons at parties or like to give them to send greetings, or for those who use them for advertising. Scientists who perform atmospheric research use helium-filled weather balloons to carry various measuring instruments into the atmosphere. You are probably familiar with the Goodyear blimp, which uses helium to stay afloat. The Goodyear blimp website contains technical information about the blimps, including their volumes and maximum gross weights. If you use the ideal gas law, the molecular weights shown above and the appropriate unit conversions, you should calculate gross weights close to those stated on the web site.
A common use of Equation 10.23 is to determine the molar mass of an unknown gas by measuring its density at a known temperature and pressure. This method is particularly useful in identifying a gas that has been produced in a reaction, and it is not difficult to carry out. A flask or glass bulb of known volume is carefully dried, evacuated, sealed, and weighed empty. It is then filled with a sample of a gas at a known temperature and pressure and reweighed. The difference in mass between the two readings is the mass of the gas.
The volume of the flask is usually determined by weighing the flask when empty and when filled with a liquid of known density such as water. The use of density measurements to calculate molar masses is illustrated in Example 10. The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given amount of gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law).
Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). A common use of Equation 6.3.9 is to determine the molar mass of an unknown gas by measuring its density at a known temperature and pressure. It is then filled with a sample of a gas at a known temperature and pressure and re-weighed.
The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables . It also allows us to predict the final state of a sample of a gas (i.e., its final temperature, pressure, volume, and amount) following any changes in conditions if the parameters are specified for an initial state. Some applications are illustrated in the following examples.
The approach used throughout is always to start with the same equation—the ideal gas law—and then determine which quantities are given and which need to be calculated. Let's begin with simple cases in which we are given three of the four parameters needed for a complete physical description of a gaseous sample. Note that every molecule listed is heavier that or equal to 18 u. Now, let's add some water vapor molecules to the gas with the total atomic weight of 18 u (H₂O - two atoms of hydrogen 1 u and one oxygen 16 u).
According to Avogadro's law, the total number of molecules remains the same in the container under the same conditions . It means that water vapor molecules have to replace nitrogen, oxygen or argon. Because molecules of H₂O are lighter than the other gases, the total mass of the gas decreases, decreasing the density of the air too. The calculator below can be used to calculate the air density and specific weight at given temperatures and atmospheric pressure. To get a better idea of the density of air specifically, you need to account for how air is made of different gases when formulating its density. At a constant temperature, pressure and volume, dry air is typically made of 78% nitrogen (N2), 21% oxygen (O2) and one percent argon (Ar).
The behavior of GHGs and other substances in this system can be to some extent predicted from the inherent properties of the compound of concern. For example, vapor pressure is the pressure exerted by a vapor in a confined space. Similarly, vaporization is the change of a liquid or solid to the vapor phase.
This means that if a substance vaporizes, it can enter the atmosphere. Thus, volatilization is a function of the concentration of a contaminant in solution and the contaminant's partial pressure. For any ideal gas, at a given temperature and pressure, the number of molecules is constant for a particular volume (see Avogadro's Law). So when water molecules are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing. Hence the mass per unit volume of the gas decreases.
Ocean uptake of anthropogenic CO2 is primarily a physical response to increasing atmospheric CO2 concentrations. Increasing the partial pressure of a gas in the atmosphere directly above the body of water causes the gas to diffuse into that water until the partial pressures across the air-water interface are equilibrated. The effects are complex, for example, increasing CO2 also modifies the climate which in turn may change ocean circulation, which changes the rate of ocean CO2 uptake.
Bioconcentration can vary considerably in the environment. The degree to which a contaminant builds up in an ecosystem, especially in biota and sediments, is related to the compound's persistence. For example, a highly persistent compound often possesses chemical structures that are also conducive to sequestration by fauna. Such compounds are generally quite often lipophilic, have high Kow values, and usually low vapor pressures. This means that they may bind to the organic molecules in living tissues and may resist elimination and metabolic process, so that they build up over time.
However, the bioaccumulation and bioconcentration can vary considerably, both among biota and within the same species of biota. For example, the pesticide mirex has been shown to exhibit BCFs of 2600 and 51,400 in pink shrimp and fathead minnows, respectively. The pesticide endrin has shown an even larger interspecies variability in BCF values, with factors ranging from 14 to 18,000 recorded in fish after continuous exposure. Intraspecies BCF ranges may also be high, e.g. oysters exposed to very low concentrations of the organometallic compound, tributyl tin, exhibit BCF values ranging from 1000 to 6000.
The properties of N2 will deviate more from ideality at -100oC than at 100oC. Van der Waal's equation corrects for the non-ideality of real gases. Molecules of CH4 at high pressures and low temperatures have no attractive forces between each other. Molecules of an ideal gas are assumed to have no significant volume. 15.Which one of the following statements is not consistent with the kinetic-molecular theory of gases?
The actual volume of the gas molecules themselves is very small compared to the volume occupied by the gas at ordinary temperatures and pressures. The average kinetic energies of different gases are different at the same temperature. There is no net gain or loss of the total kinetic energy in collisions between gas molecules. The theory explains most of the observed behavior of gases at ordinary temperatures and pressures.
The reaction of a copper penny with nitric acid results in the formation of a red-brown gaseous compound containing nitrogen and oxygen. A sample of the gas at a pressure of 727 mmHg and a temperature of 18°C weighs 0.289 g in a flask with a volume of 157.0 mL. Calculate the molar mass of the gas and suggest a reasonable chemical formula for the compound.
Relates the pressure, volume, temperature and number of moles in a gas to each other. The ideal gas law is what is called an equation of state because it is a complete description of the gas's thermodynamic state. No other information is needed to calculate any other thermodynamic variable, and, since the equation relates four variables, a knowledge of any three of them is sufficient. In which ρ is density in units of m/V mass/volume (kg/m3). Keep in mind this version of the ideal gas law uses the R gas constant in units of mass, not moles. Air concentrations at ppm are parts per million by volume and should therefore be expressed as ppmv.
Although sometimes people forget the "v" at the end, it is implied. The density of humid air can be calculated as the sum of the densities of the two gases, dry air and water vapour in proportion with their partial pressures. The gas constant is measure of the weight of the number of molecules of a gas. In the atmosphere around us we have air which is about 78% Nitrogen , about 21% Oxygen and about 1% other gases.
Nitrogen has a molecular weight of 14 so a N2 molecule has a molecular weight of 28. Oxygen has a molecular weight of 16 so an O2 molecule has a molecular weight of 32. Given the mixture of gases found in air the average molecular weight of air is around 29. Is defined as a hypothetical gaseous substance whose behavior is independent of attractive and repulsive forces and can be completely described by the ideal gas law. In reality, there is no such thing as an ideal gas, but an ideal gas is a useful conceptual model that allows us to understand how gases respond to changing conditions.
As we shall see, under many conditions, most real gases exhibit behavior that closely approximates that of an ideal gas. The ideal gas law can therefore be used to predict the behavior of real gases under most conditions. As you will learn in Section 10.8 "The Behavior of Real Gases", the ideal gas law does not work well at very low temperatures or very high pressures, where deviations from ideal behavior are most commonly observed. The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T.























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.